Introduction: The combination therapy of Venetoclax (Ven) and Azacitidine (Aza) has shown remarkable improvements in the treatment of acute myeloid leukemia (AML) patients not eligible for chemotherapy. Nevertheless, patients with TP53-mutated AML are more prone to have a refractory disease or develop rapid resistance. A recent study indicated that a combined expression ratio of BCL-2, BCL-XL and MCL-1 proteins in leukemic stem cells predicts clinical response to Ven+Aza (Waclawiczek et al. 2023). Furthermore, our previous work has demonstrated that erythroid and megakaryoblastic leukemia blasts are more dependent on BCL-XL than BCL-2 (Kuusanmäki et al. 2023). Emerging evidence suggests that the erythroid-biased differentiation and gene signature is enhanced in many TP53-mutated patients (Rodriquez-Meira et al. 2022). Here we hypothesized that in TP53 -mutated AML, leukemic blasts correspond more closely to megakaryocyte-erythroid progenitors (MEPs) leading to an unfavorable BCL-2 family expression ratio, which can be associated with poor responses to Ven. Consequently, we aimed to identify novel compounds targeting this specific patient subgroup.
Methods: We utilized flow cytometry to analyze the mean fluorescent intensity (MFI) of BCL-2 family proteins (BCL-2, BCL-XL, MCL-1) in the CD34+ blast cells of 16 bone marrow-derived AML samples taken before Ven+Aza therapy ( TP53mut n=8, TP53wt n=8). Ex vivo drug testing was performed with eight compounds and blast-specific drug responses were analyzed after 48 hours with flow cytometry. RNA sequencing was performed on CD34+ or CD117+ cells enriched from 35 pre-Ven+Aza treatment samples. To assess erythroid signature scores, we calculated the mean expression of six different erythroid genes ( GATA1, KLF1, ZFPM1, GATA2, GYPA, TFRC) for each patient, following the approach by Rodriquez-Meira et al. 2022. The expression levels of 19 intracellular proteins, including BCL-2, BCL-XL, MCL-1, were quantified from the corresponding cell population of the samples by mass cytometry (CyTOF).
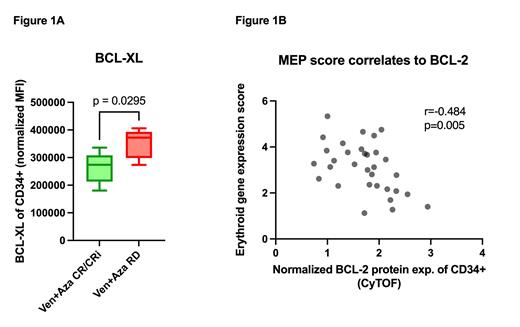
Results: In the comparison of BCL-2 family protein expression and treatment responses, BCL-XL levels were significantly higher (p=0.03) in the treatment-resistant group, while no differences were found in BCL-2 or MCL-1 ( Figure 1A). In the TP53-mutated blasts, a trend of decreased BCL-2 was identified compared to the TP53 wild-typeblasts (p=0.08), but no difference in BCL-XL or MCL-1 levels was noted. However, all three Ven+Aza-refractory TP53-mutants exhibited high BCL-XL expression compared to the treatment-responsive TP53-mutated patients.
Next, we assessed whether an erythroid-like phenotype was associated with TP53 mutation status by computing the erythroid gene signature scores from the RNAseq data of enriched blasts. Erythroid scores were increased among TP53-mutated samples (p=0.04), and interestingly, a higher erythroid score was linked to lower BCL-2 protein expression, regardless of TP53 mutation status ( Figure 1B). Finally, we assessed the drug efficacy of eight pre-selected compounds, including BCL-2 family inhibitors. As expected, Ven was ineffective in the TP53-mutated samples. In contrast, the TP53-mutated blasts showed higher ex vivo sensitivity to Navitoclax (BCL-2/BCL-XL inhibitor) (Nav IC50=90nM, Ven IC50>1000nM). Additionally, LCL-161 (IAPs inhibitor) demonstrated notable efficacy in the TP53-mutants and was significantly more effective compared to the wild-type controls (LCL-161 IC50=300nM, Ven IC50>1000nM).
Discussion: Here, we show that increased BCL-XL protein expression is associated with clinical resistance to Ven+Aza. Furthermore, the link between erythroid gene signature and BCL-2 expression suggests that especially those AML blasts harboring TP53 mutations might have impaired BCL-2 expression due to a closer resemblance to MEPs. The increased sensitivity of TP53-mutated AML cells to LCL-161 demonstrated in the study, highlights the potential of targeting extrinsic apoptosis pathway via inhibiting the inhibitors of apoptosis proteins (IAPs). Furthermore, co-targeting of BCL-2/BCL-XL might provide alternative therapeutic approach in Ven resistant TP53-mutated patients. These findings are currently being validated with a larger patient cohort.
Disclosures
Pyörälä:Faron Pharmaceuticals: Consultancy. Rimpiläinen:Faron Pharmaceuticals: Consultancy. Siitonen:Faron Pharmaceuticals: Consultancy. Gjertsen:BerGenBio: Consultancy; Coegin: Consultancy; GreinDX: Consultancy; Immedica: Consultancy; InCyte: Consultancy; Mendus AB: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Otsuka: Consultancy; Pfizer: Consultancy, Research Funding; Sanofi: Consultancy; in Alden Cancer Therapy AS: Current holder of stock options in a privately-held company; KinN Therapeutics AS: Current holder of stock options in a privately-held company. Heckman:Zentalis Pharmaceuticals: Research Funding; Autolus: Consultancy; WNTResearch: Research Funding; Oncopeptides: Research Funding; Novartis: Research Funding; Kronos Bio: Research Funding; Amgen: Honoraria. Kontro:Astellas: Consultancy; Pfizer: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Faron Pharmaceuticals: Consultancy; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Speakers Bureau; Novartis: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal